Writing Equations for Standard Enthalpy of…
How to write a balanced chemical equation for the…
Writing Equations for Standard Enthalpy of…
ChemTeam: Hess Law - using standard enthalpies of…
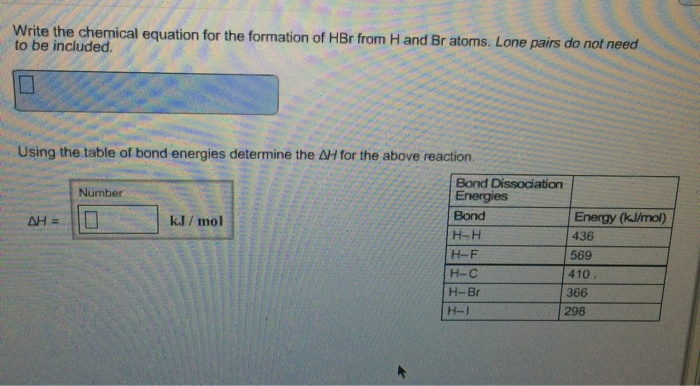

 Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
 Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
 Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
Standard Enthalpy of Formation - Chemistry…
Feb 2017 The equation for the standard enthalpy change of formation (originating from Write the chemical equation for the formation of CO2
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
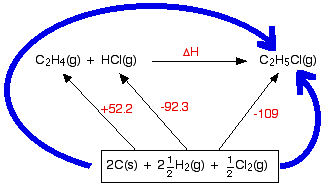 Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
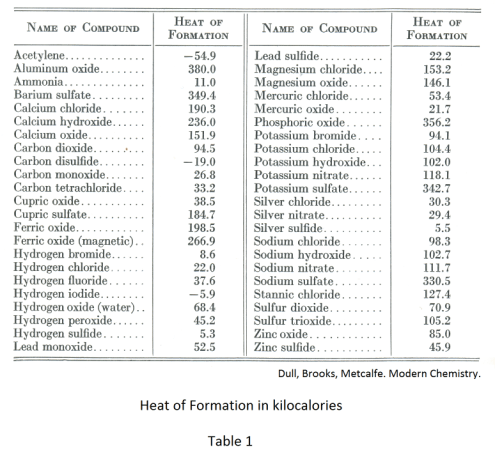 Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
How to write a balanced chemical equation for the…
Oct 2014 All substances must be in their most stable states at 100 kPa and 25 °C So we must write a thermochemical equation for the formation of 1 mol
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Formation Reactions - 2012 Book Archive
Write formation reactions for each of the following FeO(s); C 2H 6(g) Solution In both cases, there is one mole of the substance as product, and the coefficients
How to write a balanced chemical equation for the…
Oct 2014 All substances must be in their most stable states at 100 kPa and 25 °C So we must write a thermochemical equation for the formation of 1 mol
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
 Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
How to write a balanced chemical equation for the…
Oct 2014 All substances must be in their most stable states at 100 kPa and 25 °C So we must write a thermochemical equation for the formation of 1 mol
Heat of formation (video) | Enthalpy | Khan…
Standard heat of formation or standard enthalpy change of formation For example, ethane would have 6 hydrogen and 2 carbon, propane would have 8 hydrogen and 3 How do you tell whether an equation is endothermic or exothermic?
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
Enthalpies of Reaction
Calculate the enthalpies of reactions from enthalpies of formation or write the chemical reaction equations accompanying the thermodynamic values, you will
Standard Enthalpy of Formation - Chemteam info
An example: the enthalpy of formation for Br2 in its standard state is zero All chemical reactions involve a change in enthalpy (defined as the heat produced or
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
How to write a balanced chemical equation for the…
Oct 2014 All substances must be in their most stable states at 100 kPa and 25 °C So we must write a thermochemical equation for the formation of 1 mol
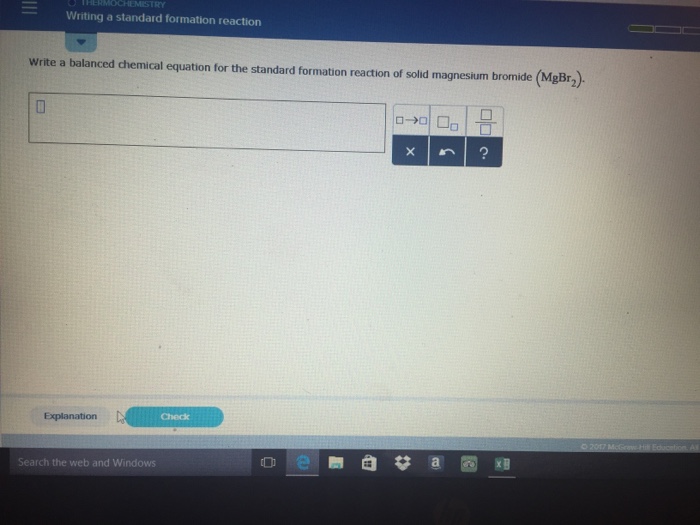
 Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem
Writing Equations for Standard Enthalpy of…
Oct 2014 How to write chemical equations for the formation of one mole of a substance from elements in their standard states
Standard Enthalpies of Formation -…
Write the reactant elements in their standard states on the ((; Balance the equation for only one mole of product For example: Write the formation reaction for
ChemTeam: Hess Law - using standard enthalpies of…
All it means is that we are discussing the enthalpy of a generic reaction, not any specific one I ll explain the above equation using an example problem